Design, synthesis, and in vitro and in vivo anticancer activity studies of new (S)-Naproxen thiosemicarbazide/1,2,4-triazole derivatives†
Abstract
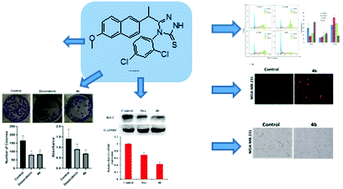
In this study, a series of novel (S)-Naproxen derivatives bearing a thiosemicarbazide/1,2,4-triazole moiety were designed, synthesized, and evaluated for anticancer activity. The structures of these compounds were characterized by spectral (1H–13C NMR, FT-IR, and HR-MS analyses) methods. All of the synthesized compounds (3a–m, 4a–j) were screened for anticancer activity against human breast cancer cell line MDA-MB-231. Among them, (S)-4-(2,4-dichlorophenyl)-5-[1-(6-methoxynaphthalen-2-yl)ethyl]-4H-1,2,4-triazole-3-thione (4b) showed the most potent anticancer activity with a good selectivity (IC50= 9.89 ± 2.4 μM). Inhibition of anti-apoptotic protein Bcl-2 was investigated in MDA-MB-231 cells treated with compound 4b using Western Blotting. Apoptosis was also detected by AO/EB and JC-1 staining. Furthermore, activation of caspase-3 enzyme activity demonstrated apoptosis. The flow cytometric analysis results showed that compound 4b decreases the number of cells in the G2/M phase and increases the cells in the S phase in a dose-dependent manner. The anticancer activity of compound 4b was also investigated. In the Ehrlich acid tumor model, a well-validated in vivo ectopic breast cancer model, compound 4b had anticancer activity and reduced the tumor volume at both low (60 mg kg−1) and high (120 mg kg−1) doses in mice, according to our in vivo results.


 Please wait while we load your content...
Please wait while we load your content...