Highly water-soluble dimeric and trimeric lanthanide carbonates with ethylenediaminetetraacetates as precursors of catalysts for the oxidative coupling reaction of methane†
Abstract
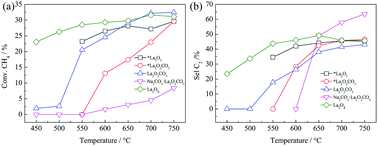
Reactions of carbon dioxide with lanthanide ethylenediaminetetraacetates M[Ln(EDTA)(H2O)3]·5H2O (M = NH4, Na or K; Ln = La or Ce) result in the formation of highly water-soluble lanthanide carbonates with ethylenediaminetetraacetates (NH4)5[La3(CO3)(EDTA)3(H2O)3]·12H2O (1), Na8[Ln2(CO3)3(EDTA)2]·17.5H2O (Ln = La, 2; Ce, 3), and K5[Ln3(CO3)(EDTA)3(H2O)3]·13.5H2O (Ln = La, 4; Ce, 5; H4EDTA = ethylenediaminetetraacetic acid) in dimethylformamide–water solution, which have been fully characterized. The main structures of these five complexes are all based on the [Ln(EDTA)]− unit, where carbonates participate due to coordination in μ1:η2(O,O) and μ2:η2(O,O′):η2(O,O′) fashion. The reactions of lanthanum ethylenediaminetetraacetate with CO2 have been monitored via13C NMR measurements. Furthermore, the calcined products of 1 and 2 are used for the oxidative coupling reaction of methane (OCM). The results demonstrate the good catalytic performances of La2O3 or La2O2CO3 from 1 and a mixture of La2O2CO3 and Na2CO3 from 2. The coordination modes of the lanthanide carbonates in precursors 1 and 2 may provide a model for the interaction of lanthanide carbonates, which is useful for the OCM reaction.


 Please wait while we load your content...
Please wait while we load your content...