Aerobic oxidation-functionalization of the aryl moiety in van Koten's pincer complex (NCN)Ni(ii)Br: relevance to carbon–heteroatom coupling reactions promoted by high-valent nickel species†
Abstract
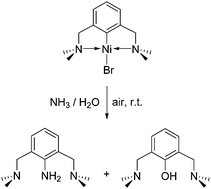
The growing popularity of metal-promoted C–H functionalization methodologies has led to increased efforts aimed at improving our understanding of the mechanistic underpinnings of C–C and C-heteroatom bond forming steps. One approach to such mechanistic studies involves probing the reactivities of model complexes featuring chelate-stabilized M-hydrocarbyl moieties. The present study uses van Koten's (NCN)Ni(II)Br compound as a model for probing C-heteroatom coupling with protic substrates HX (X = NHR, OR). Our results establish the crucial importance of aerobic (oxidative) conditions for promoting reactivities that give the functionalized ligands NC(X)N. The importance of oxidation for the C-heteroatom bond formation is further deduced from the reaction of the structurally authenticated complex (NCN)Ni(OSiMe3) with CuBr2 to give the C–O functionalization product NC(OSiMe3)N.
- This article is part of the themed collection: NJC Editorial Board web collection


 Please wait while we load your content...
Please wait while we load your content...