Catalyst-free visible light-induced decarboxylative amination of glycine derivatives with azo compounds†
Abstract
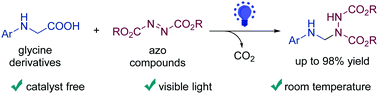
α-Amino acids such as glycine derivatives have been utilized commonly as reagents for decarboxylative functionalization. In contrast to reports revealing the requirement of transition metals and organic dyes to promote this process, we report a catalyst-free approach for the decarboxylative amination of glycine derivatives with azo compounds under visible-light irradiation. A range of important aminals was obtained with high efficiency under mild reaction conditions.


 Please wait while we load your content...
Please wait while we load your content...