Synthesis and optical properties of conjugated maleimide molecules containing amino with aggregation-induced emission enhancement (AIEE)†
Abstract
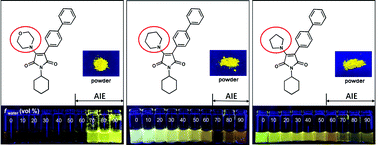
Maleimide-based luminophores bearing conjugated units are attracting intensive scientific interest as organic luminophores, owing to their excellent light emission properties and wide applications. In this article, after we synthesized amino-maleimide derivatives via a simple route, a series of amino-aryl-maleimide luminophores were synthesized by Suzuki–Miyaura coupling. The obtained luminophores show yellowish-green emission, and the emission maximum wavelength is from 534 nm to 547 nm. In particular, the obtained luminophores have large Stokes shifts (>100 nm) and the emission intensity in the solid state is stronger than that in solution with aggregation-induced emission enhancement (AIEE). In addition, the amino-aryl-maleimide luminophores respond to the increasing polarity of various solvents and show a positive solvatochromic fluorescence.


 Please wait while we load your content...
Please wait while we load your content...