Tunable Cu–M bimetal catalysts enable syngas electrosynthesis from carbon dioxide†
Abstract
The catalytic reduction of CO2 to produce syngas (CO2RR-to-syngas) gives a practicable way to utilize CO2. However, the scale relationships between the adsorption energies of the CO2 reduction intermediates severely restrict the catalytic performance of catalysts. Herein, we design Cu–M bimetal catalysts (BMCs) to break through the stubborn restriction of scaling relationships on catalytic activity. Density functional theory (DFT) was used to investigate the CO2RR activity of Cu-based BMCs. It is found that the dual-atom sites (Cu site and M site) result in noteworthy deviations for the scaling relationships between COOH* and CO*. The Cu–Cr catalyst shows the best CO2RR activity towards CO production. Specifically, only a low overpotential of 0.326 V is required to enable CO2 reduction to CO. The Cu–Rh catalyst shows a good HER activity due to the lower absolute 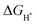 value. The electrons entering the antibonding orbital significantly increase the Pauli repulsion, which weakens the adsorption energy of atomic hydrogen on the catalysts. The d-band center (εd) is not a good descriptor for the HER and CO2RR over BMCs. Cu–W and Cu–Mo have comparable reaction free energies of the first step involved in the HER and CO2RR processes, and are expected to be the most suitable catalysts for CO2RR-to-syngas.
value. The electrons entering the antibonding orbital significantly increase the Pauli repulsion, which weakens the adsorption energy of atomic hydrogen on the catalysts. The d-band center (εd) is not a good descriptor for the HER and CO2RR over BMCs. Cu–W and Cu–Mo have comparable reaction free energies of the first step involved in the HER and CO2RR processes, and are expected to be the most suitable catalysts for CO2RR-to-syngas.



 Please wait while we load your content...
Please wait while we load your content...