Study of polyphenols from Caesalpinia paraguariensis as α-glucosidase inhibitors: kinetics and structure–activity relationship†
Abstract
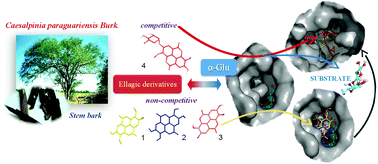
Five polyphenolic derivatives isolated from a bioactive fraction of Caesalpinia paraguariensis bark were identified as (1) ellagic acid, (2) 3-O-methylellagic, (3) 3,3′-O-dimethylellagic acid, (4) 3,3′-O-dimethylellagic-4-O-β-D-xylopyranoside and (5) (−)epigallocatechin-gallate. The yeast α-glucosidase (α-Glu) inhibitory activity was evaluated in vitro, including acarbose and luteolin as positive controls. Compound 5 showed significantly more inhibitory activity (IC50 5.20 μM) than the ellagic derivatives (IC50 65–263 μM). Compounds 3 and 5 showed non-competitive inhibition (Ki 50.0 and 7.8 μM, respectively); ellagic xyloside (4) showed competitive inhibition (Ki 17.5 μM); and ellagic acid (1) and 3-O-methylellagic (2) showed mixed-type inhibition (Ki 68.3 and 6.0 μM, respectively). Computational studies considering the experimental kinetic constants were performed by homology modelling and molecular docking to predict the binding mode of the compounds. Subsequently, a detailed analysis of the molecular interactions at the α-Glu active site was performed by molecular dynamic simulations and quantum theory of atoms in molecules (MD/QTAIM). While some expected relationships between structural and activity data were verified, a more general structure–activity relationship could not be derived because of the different types of inhibition exerted by the compounds. Therefore, we propose an alternative binding site for non-competitive inhibitors that along with active site binding provides a more comprehensive picture of the inhibition event.


 Please wait while we load your content...
Please wait while we load your content...