Molten salt-assisted synthesis of special open-cell Fe, N co-doped porous carbon as an efficient electrocatalyst for zinc–air batteries†
Abstract
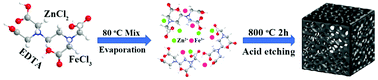
Fe, N co-doped carbon electrocatalyst is one of the most attractive alternatives to Pt/C catalysts due to its high catalytic activity, excellent stability and low cost. However, obtaining stable and efficient Fe, N co-doped carbon oxygen reduction reaction (ORR) catalysts based on simple processes is still a challenge. Herein, Fe, N co-doped porous carbon (Fe–N–C) with an open macroporous frame structure is prepared by using inorganic molten salts (FeCl3·6H2O and ZnCl2) and ethylenediaminetetraacetic acid as the template and nitrogen-containing carbon precursor, respectively. The open pore structure of the Fe–N–C material precisely tailored with the inorganic molten salt template exhibits high specific surface area (676.5 m2 g−1) and appropriate pore size, which can promote oxygen adsorption and expand the oxygen reduction interface, resulting in the acceleration of the electron/charge transfer processes and an improved electrocatalytic performance. The optimized Fe3–N–C-800 electrocatalyst exhibits good catalytic activity for ORR, such as high onset potential of 0.998 V and half wave potential of 0.84 V in alkaline media, as well as high stability and methanol tolerance. Furthermore, a novel Zn–air battery assembled with carbon paper containing Fe3–N–C-800 electrocatalyst as air cathode shows high open-circuit voltage (1.485 V), high specific capacity (870 mA h g−1 at 10 mA cm−2), excellent reversibility and stability. The proposed synthetic strategy provides new opportunities to design and construct carbon frame materials with the desired open macroporous structure to improve their electrocatalytic performance.


 Please wait while we load your content...
Please wait while we load your content...