Manipulation of electronic and structural effects on the solid-state emission of multiple linked anthracenyl-o-carborane dyads†
Abstract
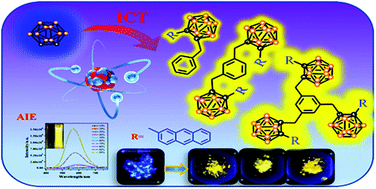
A direct boost of emission efficiency is presented based on the strategy of linking multiple weak AIE-active anthracenyl-o-carborane dyads together for improving solid-state luminescence. Multiple anthracenyl-o-carborane dyads, linked with benzyl (CA1), 1,3-bismethyl-benzene (CA2), 1,3,5-trismethyl-benzene (CA3), were prepared and fully characterized and their optical properties were compared. Various types of substituent groups at the adjacent carbon atom of o-carborane can restrict the vibration of Ccage–Ccage bonds and rotational motion of the o-carborane moiety and regulate the orientation of ICT from the electron-donating moiety to the o-carborane cluster. CA1, CA2, and CA3 are all AIE-active molecules. The emission efficiencies of CA1, CA2, and CA3 were 8- to 16-fold greater than that of anthracenyl-o-carborane dyad (ΦF = 4.3%) in the solid state, clearly indicating that the combination of multiple anthracenyl-o-carborane dyads should be valid for enhancing luminous efficiency. DFT calculations on the structures ofexcited states suggested that the ICT emission is via the twisted intramolecular charge transfer (TICT) mechanism in the multi-linked anthracenyl-o-carborane dyads.


 Please wait while we load your content...
Please wait while we load your content...