β-Aldehyde ketones as dual inhibitors of aldose reductase and α-glucosidase with antioxidant properties†
Abstract
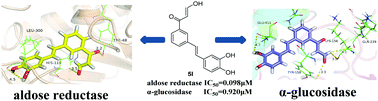
In this study, a series of phenyl β-aldehyde ketones were designed and synthesized as novel dual inhibitors of aldose reductase (ALR2) and α-glucosidase. The results showed substantial inhibition of ALR2 and α-glucosidase by the synthetic compounds and also demonstrated the antioxidant activity of these inhibitors. Among them, compound 5l had the most potent inhibitory effect on both enzymes, with non-competitive inhibition patterns, and was the most effective antioxidant in tests for 2,2-diphenyl-1-picrylhydrazyl radical scavenging and lipid peroxidation suppression. High lipophilicity and negligible cytotoxicity were also observed for this compound. Analysis indicated that the β-aldehyde ketone moiety might play a vital role in the inhibitory effect on the enzymes and that the phenolic hydroxy group was the key structure for intensifying the inhibitory and antioxidant activities. Overall, the results confirmed the successful synthesis of potent ALR2 and α-glucosidase inhibitors with antioxidant activity.


 Please wait while we load your content...
Please wait while we load your content...