Molecular design of DBA-type five-membered heterocyclic rings to achieve 200% exciton utilization for electroluminescence†
Abstract
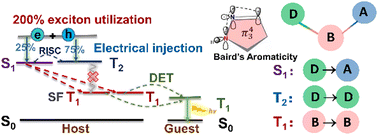
Achieving high exciton utilization is a long-cherished goal in the development of organic light-emitting diode materials. Herein, a three-step mechanism is proposed to achieve 200% exciton utilization: (i) hot triplet exciton (T2) conversion to singlet S1; (ii) singlet fission from S1 into two T1; (iii) and then a Dexter energy transfer to phosphors. The requirement is that S1 should lie slightly lower than or close to T2 and twice as high as T1 in energy. For this, a scenario is put forward to design a series of donor-bridge-acceptor (DBA) type molecules with 2E(T1) ≤ E(S1) < E(T2), in which the Baird-type aromatic pyrazoline ring is used as a bridge owing to its stabilized T1 (1.30–1.74 eV) and different kinds of donors and acceptors are linked to the bridge for regulating S1 (2.35–3.87 eV) and T2 (2.44–3.96 eV). The ultrafast spectroscopy and sensitization measurements for one compound (TPA-DBPrz) fully confirm the theoretical predictions.


 Please wait while we load your content...
Please wait while we load your content...