Tuning hydrophobic composition in terpolymer-based anion exchange membranes to balance conductivity and stability†
Abstract
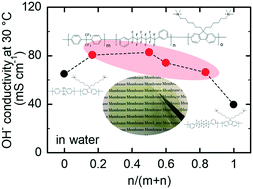
We designed and synthesized novel terpolymer-based anion conductive polymers, where the effect of hydrophobic composition on the membrane properties was investigated in detail. Precursor terpolymers were first prepared from 2,2-bis(4-chlorophenyl)hexafluoropropane (BAF), 1,6-bis(3-chlorophenyl)perfluorohexane (PAF), and 2,7-dichloro-9,9-bis[6′-(N,N-dimethylamino)hexyl]fluorene via Ni(0)-promoted polycondensation reaction. The following quaternization reaction with dimethyl sulfate was successful to obtain five terpolymers, QBPA with different PAF/(BAF + PAF) compositions and supposed chemical structures. QBPA provided thin and bendable membranes by solution casting. TEM images suggested that the membranes exhibited a phase-separated morphology similar to those of the corresponding parent copolymer membranes. SAXS profiles indicated that QBPA-4 containing 83 mol% PAF exhibited the most distinct periodic structure based on the hydrophobic component. The hydroxide ion conductivity of the membranes showed a volcano-type dependence on the hydrophobic composition, and the highest conductivity (161 mS cm−1) was achieved with the QBPA-1 membrane at 80 °C. Taking also the other properties into account, QBPA-1 and QBPA-5 containing 17 mol% PAF seemed the best-balanced membranes. An alkaline fuel cell using the QBPA-1 membrane achieved a maximum power density of 273 mW cm−2, exceeding that using the copolymer BAF-QAF membrane (185 mW cm−2).


 Please wait while we load your content...
Please wait while we load your content...