Structure–activity relationship study of PROTACs against hematopoietic prostaglandin D2 synthase†
Abstract
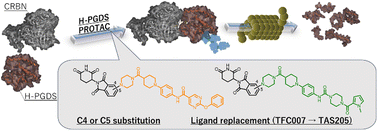
Degradation of hematopoietic prostaglandin D2 synthase (H-PGDS) by proteolysis-targeting chimeras (PROTACs) is expected to be important in the treatment of allergic diseases and Duchenne's muscular dystrophy. We recently reported that PROTAC(H-PGDS)-7 (PROTAC1), which is composed of H-PGDS inhibitor (TFC-007) and cereblon (CRBN) E3 ligase ligand (pomalidomide), showed potent H-PGDS degradation activity. Here, we investigated the structure–activity relationships of PROTAC1, focusing on the C4- or C5-conjugation of pomalidomide, in addition, the H-PGDS ligand exchanging from TFC-007 with the biaryl ether to TAS-205 with the pyrrole. Three new PROTACs were evaluated for H-PGDS affinity, H-PGDS degrading activity, and inhibition of prostaglandin D2 production. All compounds showed high H-PGDS degrading activities, but PROTAC(H-PGDS)-4-TAS-205 (PROTAC3) was slightly less active than the other compounds. Molecular dynamics simulations suggested that the decrease in activity of PROTAC3 may be due to the lower stability of the CRBN-PROTAC-H-PGDS ternary complex.


 Please wait while we load your content...
Please wait while we load your content...