Efficient selective targeting of Candida CYP51 by oxadiazole derivatives designed from plant cuminaldehyde†
Abstract
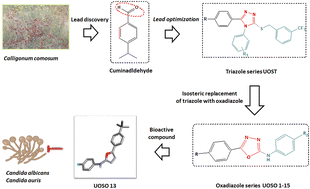
Candida infection represents a global threat with associated high resistance and mortality rate. Azoles such as the triazole drug fluconazole are the frontline therapy against invasive fungal infections; however, the emerging multidrug-resistant strains limit their use. Therefore, a series of novel azole UOSO1–15 derivatives were developed based on a modified natural scaffold to combat the evolved resistance mechanism and to provide improved safety and target selectivity. The antifungal screening against C. albicans and C. auris showed that UOSO10 and 12–14 compounds were the most potent derivatives. Among them, UOSO13 exhibited superior potent activity with MIC50 values of 0.5 and 0.8 μg mL−1 against C. albicans and C. auris compared to 25 and 600 μg mL−1 for fluconazole, respectively. UOSO13 displayed significant CaCYP51 enzyme inhibition activity in a concentration-dependent manner with an IC50 10-fold that of fluconazole, while exhibiting no activity against human CYP50 enzyme or toxicity to human cells. Furthermore, UOSO13 caused a significant reduction of Candida ergosterol content by 70.3% compared to a 35.6% reduction by fluconazole. Homology modeling, molecular docking, and molecular dynamics simulations of C. auris CYP51 enzyme indicated the stability and superiority of UOSO13. ADME prediction indicated that UOSO13 fulfils the drug-likeness criteria with good physicochemical properties.


 Please wait while we load your content...
Please wait while we load your content...