Novel benzimidazole-based pseudo-irreversible butyrylcholinesterase inhibitors with neuroprotective activity in an Alzheimer's disease mouse model†
Abstract
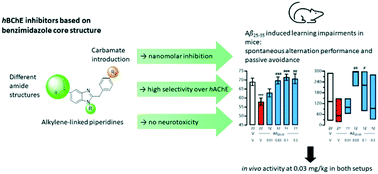
As levels of acetylcholinesterase (AChE) decrease while levels of butyrylcholinesterase (BChE) increase in later stages of Alzheimer's disease (AD), BChE stands out as a promising target for treatment of AD. Therefore, several benzimidazole-carbamates were designed based on docking studies to inhibit BChE selectively over AChE, while retaining a reasonable solubility. Synthesized molecules exhibit IC50 values from 2.4 μM down to 3.7 nM with an overall highly hBChE-selective profile of the designed compound class. After evaluation of potential neurotoxicity, the most promising compound was further investigated in vivo. Compound 11d attenuates Aβ25–35-induced learning impairments in both spontaneous alternation and passive avoidance responses at a very low dosage of 0.03 mg kg−1, proving selective BChE inhibition to lead to effective neuroprotectivity in AD.
- This article is part of the themed collection: NeuroMedChem

 Please wait while we load your content...
Please wait while we load your content...