Thiamine analogues as inhibitors of pyruvate dehydrogenase and discovery of a thiamine analogue with non-thiamine related antiplasmodial activity†
Abstract
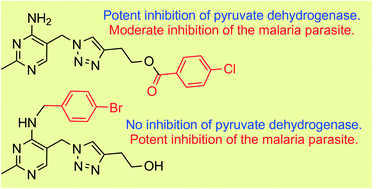
A series of derivatives of a triazole analogue of thiamine has been synthesised. When tested as inhibitors of porcine pyruvate dehydrogenase, the benzoyl ester derivatives proved to be potent thiamine pyrophosphate (TPP) competitive inhibitors, with the affinity of the most potent analogue (Ki = 54 nM) almost matching the affinity of TPP itself. When tested as antiplasmodials, most of the derivatives showed modest activity (IC50 value >60 μM), except for a 4′-N-benzyl derivative, which has an IC50 value in the low micromolar range. This activity was not affected by increasing the extracellular concentration of thiamine in the culture medium for any of the compounds (except a modest increase in the IC50 for the unfunctionalized benzoyl ester), nor by overexpressing thiamine pyrophosphokinase in the parasite, making it unlikely to be due to an effect on thiamine transport or metabolism.


 Please wait while we load your content...
Please wait while we load your content...