Design, synthesis and biological evaluation of light-driven on–off multitarget AChE and MAO-B inhibitors†
Abstract
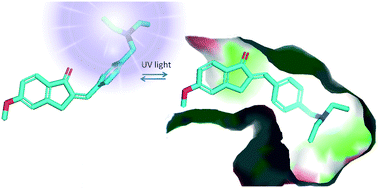
Neurodegenerative diseases are multifactorial disorders characterized by protein misfolding, oxidative stress, and neuroinflammation, finally resulting in neuronal loss and cognitive dysfunctions. Nowadays, an attractive strategy to improve the classical treatments is the development of multitarget-directed molecules able to synergistically interact with different enzymes and/or receptors. In addition, an interesting tool to refine personalized therapies may arise from the use of bioactive species able to modify their activity as a result of light irradiation. To this aim, we designed and synthesized a small library of cinnamic acid-inspired isomeric compounds with light modulated activity able to inhibit acetylcholinesterase (AChE) and monoamine oxidase B (MAO-B), with remarkable selectivity over butyrylcholinesterase (BChE) and MAO-A, which have been investigated as the enzyme targets related to Alzheimer's disease (AD). The inhibitory activities were evaluated for the pure E-diastereomers and the E/Z-diastereomer mixtures, obtained upon UV irradiation. Molecular docking studies were carried out to rationalize the differences in the inhibition potency of the E and Z diastereomers of the best performing analogue 1c. Our preliminary findings may open-up the way for developing innovative multitarget photo-switch drugs against neurodegenerative diseases.


 Please wait while we load your content...
Please wait while we load your content...