Synthesis of a fluorinated pyronin that enables blue light to rapidly depolarize mitochondria†
Abstract
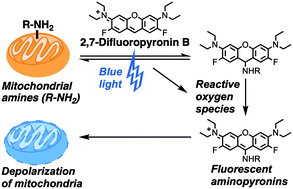
Fluorinated analogues of the fluorophore pyronin B were synthesized as a new class of amine-reactive drug-like small molecules. In water, 2,7-difluoropyronin B was found to reversibly react with primary amines to form covalent adducts. When this fluorinated analogue is added to proteins, these adducts undergo additional oxidation to yield fluorescent 9-aminopyronins. Irradiation with visible blue light enhances this oxidation step, providing a photochemical method to modify the biological properties of reactive amines. In living HeLa cells, 2,7-difluoropyronin B becomes localized in mitochondria, where it is partially transformed into fluorescent aminopyronins, as detected by spectral profiling confocal microscopy. Further excitation of these cells with the blue laser of a confocal microscope can depolarize mitochondria within seconds. This biological activity was only observed with 2,7-difluoropyronin B and was not detected with analogues such as pyronin B or 9-methyl-2,7-difluoropyronin B. This irradiation with blue light enhances the cellular production of reactive oxygen species (ROS), suggesting that increased ROS in mitochondria promotes the formation of aminopyronins that inactivate biomolecules critical for maintenance of mitochondrial membrane potential. The unique reactivity of 2,7-difluoropyronin B offers a novel tool for photochemical control of mitochondrial biology.


 Please wait while we load your content...
Please wait while we load your content...