Effects of cation vacancies at tetrahedral sites in cobalt spinel oxides on oxygen evolution catalysis†
Abstract
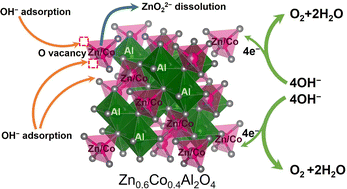
Cobalt spinel oxides exhibit high catalytic activity for the oxygen evolution reaction (OER). Therefore, accurately identifying the effects of ions from different sites on OER catalysis is essential to improve catalytic activity. Co ions at octahedral sites are usually considered as active sites, whereas those at tetrahedral sites are less active. In this study, the effects of cation vacancies at the tetrahedral sites were investigated for the OER, excluding the contributions from the active cations at the octahedral sites, using a series of Zn-substituted cobalt spinel oxides, ZnxCo1−xAl2O4 (x = 0, 0.2, 0.4, 0.6, 0.8, and 1), where Co ions occupy only the tetrahedral sites because Al ions tend to occupy the octahedral sites. Among them, Zn0.6Co0.4Al2O4 exhibited the highest OER activity and comparable kinetics to that of Co3O4, which is attributed to the cation vacancies generated by Zn dissolution. The Al ions in the structure enhanced the stability of the cobalt spinel oxides, and the spinel structure of Zn0.6Co0.4Al2O4 was retained after 1200 OER cycles. By excluding the active sites from the octahedral sites, this study demonstrates that a certain degree of cation vacancies at the tetrahedral sites can effectively improve the activity of less active Co ions at the tetrahedral sites, thus providing an important basis for future catalyst design.


 Please wait while we load your content...
Please wait while we load your content...