Sol–gel synthesis of alumina gel@zeolite X nanocomposites for high performance water defluoridation: batch and column adsorption study†
Abstract
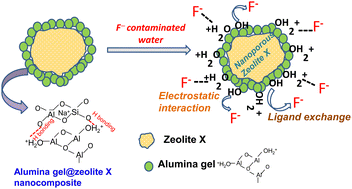
Fluoride content in groundwater above the permissible limit is a major concern worldwide due to its detrimental effects toward human beings. The development of a suitable adsorbent for water defluoridation with high efficiency still remains a great challenge. In this work, an alumina gel@zeolite X nanocomposite was prepared by modification of rice husk ash derived zeolite X with sol–gel derived alumina sol at 70 °C/2 h. The crystallinity of zeolite X and the presence of the Si–O–Al vibrational band in the nanocomposite material were confirmed by X-ray diffraction (XRD) and Fourier transform infrared spectroscopy (FTIR), respectively. The presence of the adsorbed hydroxyl (OH−) group was confirmed by an X-ray photoelectron spectroscopy (XPS) study indicating the binding energy (BE) at 533.2 eV of the O 1s spectra. During the adsorption process an electrostatic force of attraction and ion exchange mechanism occur between protonated hydroxyl/surface hydroxyl groups and fluoride ions (F−). The nanocomposite with a BET surface area of 257 m2 g−1 shows ≥99% fluoride removal for a 1–2 g L−1 adsorbent dose. In the batch study, the Langmuir model was found to be the best fitted adsorption isotherm showing the maximum adsorption capacity of 103.6 mg g−1 for the adsorbent doses of 0.5 g L−1, while in the column study, the bed depth service time (BDST) model shows an adsorption capacity of 2933.55 mg L−1.


 Please wait while we load your content...
Please wait while we load your content...