Electron density regulation of Pt–Co nanoalloys via P incorporation towards methanol electrooxidation†
Abstract
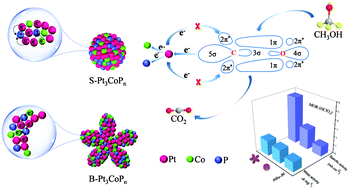
The activity of Pt catalysts for the electro-oxidation of methanol can be greatly enhanced by coupling the electron effect with morphological engineering. Herein, we report the use of bimetallic Pt–Co alloys with different molar ratios/morphologies as precursors for P doping to prepare high-efficiency electrocatalysts for the methanol oxidation reaction. We will demonstrate that the P doping could not only change the electron density around Pt atoms, but also significantly alter the original morphology of the alloy precursors. The strong electron affinity of P decreases the electron cloud density around Pt atoms, favorable for weakening the adsorption of poisonous CO-like intermediates and thus enhancing their electrocatalytic activity for methanol oxidation. In particular, the as-prepared ternary Pt3CoPn nanoparticles with branched morphologies exhibit a specific activity and mass activity for methanol oxidation of up to 5.62 mA cm−2 and 1790 mA mg−1, respectively, which are 2.3 and 1.1 times those of ternary Pt3CoPn spheres, 4.8 and 1.5 times those of commercial PtRu/C catalysts and surpass those of most Pt-based electrocatalysts reported in the literature. This study thus offers a vivid example to demonstrate the alloying of a transition metal and a non-metal element into active Pt catalysts for improving their electrocatalytic properties.


 Please wait while we load your content...
Please wait while we load your content...