A novel ion-responsive photonic hydrogel sensor for portable visual detection and timely removal of lead ions in water†
Abstract
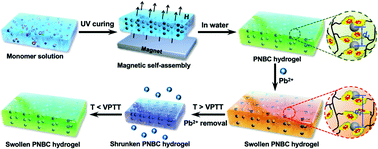
Lead-ion (Pb2+) contamination is a serious environmental problem worldwide. Although various methods have been developed for the detection or removal of Pb2+ in water environments, the availability of techniques with the capability of both highly selective, visual monitoring and timely separation remains a challenge. Herein we report a novel ion-responsive photonic hydrogel sensor based on the poly(N-isopropylacrylamide-co-benzo-18-crown-6-acrylamide) (PNBC) hydrogel with embedded orderly Fe3O4 colloidal nanocrystal cluster (CNC) chains, for highly selective and portable visual detection of Pb2+ by the naked eye, and the effective elimination of Pb2+ in aqueous solutions. The pendent 18-crown-6 units in the hydrogel backbone act as ion-responsive receptors to form B18C6Am/Pb2+ host–guest inclusion complexes with Pb2+ in water, while the poly(N-isopropylacrylamide) (PNIPAM) moieties serve as thermosensitive actuators to regulate the inclusion constants of the B18C6Am/Pb2+ complexes, which significantly affect the volume of the hydrogel. The fixed Fe3O4 CNC chains function as optical signal transmitters that can diffract visible light, thus endowing the hydrogel with bright structural colors. The osmotic pressure and swelling of the PNBC hydrogel can induce an increase in lattice spacing of the Fe3O4 CNCs when Pb2+ is selectively captured by the 18-crown-6 units, thus triggering a redshift in the diffraction wavelength and showing structural color changes of the PNBC hydrogel according to the Bragg diffraction law. Correspondingly, highly selective Pb2+ detection can be realized by the Pb2+-induced diffraction-wavelength shifts or direct observation of the color changes of the hydrogel by the naked eye. Furthermore, such a functional hydrogel sensor can simultaneously adsorb Pb2+ from aqueous solution and the Freundlich isotherm model fits well with the adsorption process. Most importantly, the facile and complete regeneration of the photonic hydrogel sensor can be easily achieved by simply changing the operating temperature to release the bound Pb2+ from the 18-crown-6 units based on the excellent thermosensitivity of the PNIPAM moieties. Such a novel photonic hydrogel sensor with high selectivity, portable visual detection and an excellent removal performance toward Pb2+ in water, as well as excellent regenerability, holds great promise for the monitoring and remediation of environmental pollutants.


 Please wait while we load your content...
Please wait while we load your content...