Synthesis of acrylonitrile side chain-appended π-conjugated polymers by a Suzuki cross-coupling polycondensation and a Knoevenagel condensation, and their optical properties†
Abstract
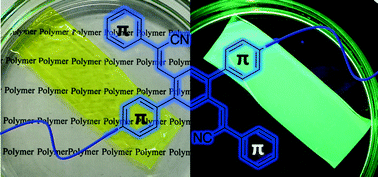
Acrylonitrile is a highly valuable unit for the design of donor–acceptor systems and luminescent π-conjugated molecular materials. Acrylonitrile-based polymers are fundamental structures in π-conjugated polymers. In contrast to the frequently reported introduction of acrylonitrile units into the main chain, we report herein their introduction into the side chain. In the present study, we carried out two syntheses: (1) a post-polymerization modification reaction in which polymers with acrylonitrile units in their side chains were produced via a Knoevenagel condensation between a π-conjugated polymer containing aldehyde groups and a cyano group-containing molecule; and (2) a one-pot process comprising a simultaneous Suzuki cross-coupling polycondensation and a Knoevenagel condensation. We obtained a high-molecular-weight π-conjugated polymer with an acrylonitrile side chain that we used to fabricate a free-standing film. The π-conjugated polymer with alkoxyphenyl acrylonitrile in its side chains exhibited charge transfer interaction based on its donor–acceptor system. It also demonstrated favourable fluorescence properties as a solid or in solution.


 Please wait while we load your content...
Please wait while we load your content...