Study on the different mechanoluminescence of anthracene derivatives†
Abstract
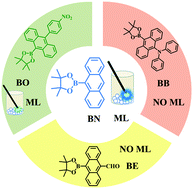
The mechanoluminescence (ML) properties of anthracene derivatives were studied. The fountainhead molecule is a plain compound BN, consisting of anthracene and boric acid ester. Then, by introducing three simple substituent groups to BN, three derivatives (BO, BE and BB) were obtained to adjust the packing modes and intermolecular interactions of the molecules simultaneously. Among these four compounds, BN presented bright blue ML observed under daylight and could maintain ML for more than 2 min by constant grinding. The molecule BO substituted by an electron-withdrawing nitrobenzene group showed green weak ML. However, the molecules BE substituted by an electron-withdrawing formyl unit and BB substituted by an electron-donating diphenylamine unit displayed no ML. Furthermore, according to the crystal analysis and DFT calculations, the preliminary relationship between the ML properties and molecular structure was summarized. A feasible design strategy of anthracene derivatives with ML is proposed.


 Please wait while we load your content...
Please wait while we load your content...