Aminoglycoside-induced lipotoxicity and its reversal in kidney on chip†
Abstract
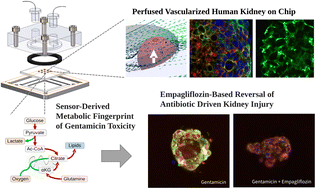
Aminoglycosides are an important class of antibiotics that play a critical role in the treatment of life-threatening infections, but their use is limited by their toxicity. In fact, gentamicin causes severe nephrotoxicity in 17% of hospitalized patients. The kidney proximal tubule is particularly vulnerable to drug-induced nephrotoxicity due to its role in drug transport. In this work, we developed a perfused vascularized model of human kidney tubuloids integrated with tissue-embedded microsensors that track the metabolic dynamics of aminoglycoside-induced renal toxicity in real time. Our model shows that gentamicin disrupts proximal tubule polarity at concentrations 20-fold below its TC50, leading to a 3.2-fold increase in glucose uptake, and reverse TCA cycle flux culminating in a 40-fold increase in lipid accumulation. Blocking glucose reabsorption using the SGLT2 inhibitor empagliflozin significantly reduced gentamicin toxicity by 10-fold. These results demonstrate the utility of sensor-integrated kidney-on-chip platforms to rapidly identify new metabolic mechanisms that may underly adverse drug reactions. The results should improve our ability to modulate the toxicity of novel aminoglycosides.


 Please wait while we load your content...
Please wait while we load your content...