Electrochemically driven oxidative C–H/N–H cross-coupling reactions of cyclic sulfamidate imines with primary anilines and secondary amines†
Abstract
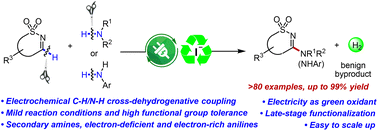
An electrosynthetic approach was developed for the oxidative C–H/N–H cross-coupling amination of cyclic sulfamidate imines under undivided electrolytic conditions via an atom- and step-economic method. In this transformation, a wide variety of cyclic or acyclic dialkyl amines, amino acid derivatives, and more challenging primary anilines acted as suitable substrates with good reaction outcomes. The synthetic advantage of this electrochemical method is augmented by high functional group compatibility (86 examples), ease of scale-up to gram scale, and accomplishment of late-stage functionalization of complex biologically relevant molecules, which provides an efficient tool with broad potential application prospects in medicinal and synthesis chemistry. Mechanistic studies reveal that this transformation probably occurred through a radical process.


 Please wait while we load your content...
Please wait while we load your content...