Photoinduced radical cascade reactions for the thioalkylation of quinoxalin-2(1H)-ones: an access to β-heteroaryl thioethers under metal- and catalyst-free conditions†
Abstract
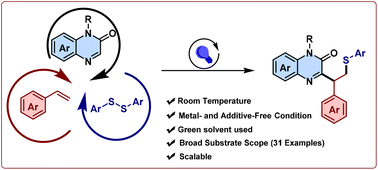
The key significance of the radical cascade process is the ease of building complex organic frameworks. As a result, introducing pharmaceutically important heterocycles to cascade reactions for their regioselective functionalization in an environmentally friendly manner has been challenging yet desirable. Here, we developed an efficient and green strategy for the thioalkylation of quinoxalin-2(1H)-ones with alkenes and aryl disulfides mediated by visible light. Our protocol showed viability with various functionalities on quinoxalin-2(1H)-ones, alkenes, and aryl disulfides. This methodology was also amenable to alkyne derivatives to deliver trisubstituted thioalkenylated quinoxalin-2-ones. Mechanistic studies showcased an interesting involvement of quinoxalin-2(1H)-ones as a photosensitizer, which obviates the requirement of an external photocatalyst.


 Please wait while we load your content...
Please wait while we load your content...