Substituent-controlled selective synthesis of 1,2-diketones and internal alkynes from terminal alkynes and arylboronic acids via α-stilbene radicals obtained from heteroleptic Cu(i) complexes under visible light†
Abstract
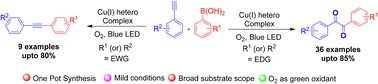
Herein, we report a substituent-controlled synthesis of 1,2-diketones and internal alkynes from terminal alkynes and arylboronic acids via α-stilbene radicals obtained from heteroleptic Cu(I) complexes under visible-light irradiation. The in situ generated α-stilbene radical Cu(II)-complex was achieved by photoinduced Csp–Csp2 coupling of copper(I) acetylides and aryl radicals catalysed by heteroleptic Cu(I) complexes. This photochemical approach offered high atom economy with a low E-factor and functional group tolerance under mild reaction conditions. Mechanistic insights clearly show that the reaction proceeded via copper(I) acetylides and the phenyl radical intermediate pathway.


 Please wait while we load your content...
Please wait while we load your content...