Olefin epoxidation using electricity as renewable power in a bromide-mediated electrochemical process†
Abstract
Electrochemical bromide-mediated olefin epoxidation is a viable strategy to generate freely available bromine (FAB) in situ while minimizing process waste by avoiding chemical oxidants and reducing the use of hazardous reagents. Herein, an electrochemical bromide-mediated olefin epoxidation is presented, wherein bromide plays a double role, as an electrolyte, but also as a mediator that is easily oxidized even at moderate anodic voltage. A quaternary ammonium salt is introduced as an organic electrolyte. The amount of applied charge and the concentrations of electrolyte and olefin were tuned to maximize the efficiency and selectivity of the reaction. Target compounds like 1,2-epoxycyclohexane were obtained in high yields of up to 93%, and a broad substrate scope was demonstrated. Additionally, this process can avoid the formation of dibrominated compounds (e.g., 1,2-dibromocyclohexane), which are typically undesirable dead-end products.
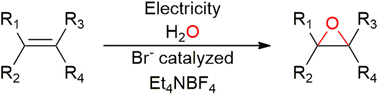
- This article is part of the themed collection: International Symposium on Green Chemistry 2022


 Please wait while we load your content...
Please wait while we load your content...