Hydrogenation of CO2 to formic acid in biphasic systems using aqueous solutions of amino acids as the product phase†
Abstract
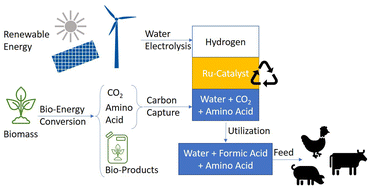
Carbon capture and utilization is considered a promising approach for introducing CO2 into the chemical value chain, especially in combination with bioenergy applications (BECCU). We report here on the catalytic hydrogenation of CO2 to formic acid in a biphasic reaction system using aqueous solutions of amino acids as the product phase and possible capture solutions for biogenic CO2. The molecular structure of the ruthenium catalyst and the catalyst phase were matched through a combined design process identifying n-dodecanol (lauryl alcohol) as the preferred “green” solvent. A total turnover number (TON) of over 100 000 mole HCOOH per mole of catalyst (46 582 g HCOOH per g of Ru) with minimal contamination of the aqueous phase with metal or organic solvent was obtained. The resulting aqueous solutions attained almost quantitative conversions with up to 0.94 mol formic acid per mol amino acid (ca. 108 g HCOOH per kg). Such solutions may find use directly, or after upgrading, in agricultural applications without the need for energy intensive and costly isolation of pure formic acid.


 Please wait while we load your content...
Please wait while we load your content...