Cathode electrolysis for the comprehensive recycling of spent lithium-ion batteries†
Abstract
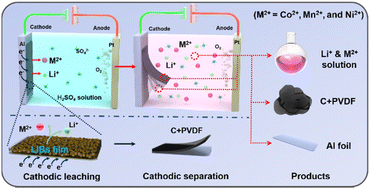
Clean lithium-ion battery recycling is indispensable to make the battery market sustainable. Here, we develop an electrochemical approach to separate the cathode film (6 cm × 20 cm) from the Al substrate and simultaneously leach Li and transition metals from the cathode film in 0.5 M H2SO4, leaving behind the conductive carbon and polyvinylidene fluoride binder that are reusable. For LiCoO2 batteries, LiCoO2 was converted to soluble Li+ and Co2+ with a leaching efficiency of over 97%. Besides, the cathode electrolysis is applicable to treat LiMn2O4 and LiNi0.5Co0.2Mn0.3O2. Overall, the cathode electrolysis employs electrons as both reducing agents and exfoliation force, offering a promising pathway to comprehensively reclaim all components from spent cathodes with limited secondary wastes and energy consumption.
- This article is part of the themed collection: 2022 Green Chemistry Hot Articles


 Please wait while we load your content...
Please wait while we load your content...