Highly efficient catalytic transfer hydrogenolysis for the conversion of Kraft lignin into bio-oil over heteropoly acids†
Abstract
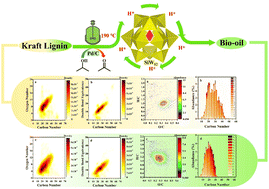
Catalytic transfer hydrogenolysis (CTH) has emerged as a sustainable and carbon-neutral biorefinery process due to the decrease in the carbon footprint and high atom and energy efficiency. Herein, heteropoly acids (HPAs) as green catalysts were used for the CTH of Kraft lignin (KL) into lignin oil under mild conditions. It is found that all the types of HPAs, Pd/C, H-donor solvent and reaction temperature affected significantly the conversion of Kraft lignin. SiW12 and PW12, respectively, afforded 95.0% and 83.8% KL conversion in isopropanol with Pd/C at 190 °C for 5 h. However, the degradation of eucalyptus Kraft lignin (EKL) provided phenolic monomers with the highest yield of 42.6% using SiW12 and Pd/C. Molecular dynamics (MD) simulation showed that SiW12 and PW12 in isopropanol are more likely to be close to lignin compared to n-propanol. The relative average molecular weight of the obtained lignin oil ranges from 100 Da to 700 Da, including phenolic monomers, dimers and trimers. The lignin monomer products are mainly composed of guaiacols and syringols with 0 to 3 carbon side chains. The simultaneous depolymerization and deoxygenation of KL during CTH led to a high-boiling organic fraction, analyzed with a Fourier transform-ion cyclotron resonance mass spectrometer (FT-ICR MS) equipped with an electrospray negative ion source (ESI−), maintaining highly aromatic structures and having lower O/C and higher H/C ratios compared to initial lignin. This work exhibits a prospective application of the CTH for the conversion of technical lignin to chemicals and biofuels.


 Please wait while we load your content...
Please wait while we load your content...