Metal/catalyst-free sequential C–N bond forming cascades at room temperature: environment-friendly one-pot synthesis of 5-aminoimidazoles from aryl glyoxals, anilines, and amidines†
Abstract
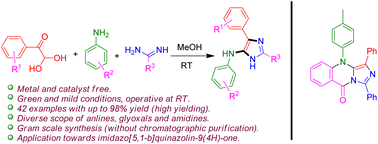
An expeditious one-pot method for the synthesis of highly substituted 5-aminoimidazoles from glyoxals, anilines, and amidines was evaluated under metal/catalyst-free conditions operative at room temperature. The present protocol constitutes an environmentally friendly approach for the synthesis of highly substituted 5-aminoimidazoles, which are important building blocks of several bioactive and medicinal compounds. The readily and commercially available starting materials can be quickly assembled into imidazoles using this protocol and scaling up this process does not require chromatographic purification. Additionally, the reaction of 5-aminoimidazole with 2-bromobenzoyl chloride can provide a novel imidazo[5,1-b]quinazolin-9(4H)-one, thus showcasing the applicability of this protocol.


 Please wait while we load your content...
Please wait while we load your content...