In-water synthesis of isocyanides under micellar conditions†
Abstract
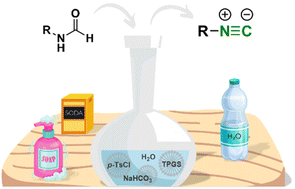
An in-water dehydration of N-formamides to afford isocyanides using micellar conditions at room temperature is reported. This method allows for the preparation of aliphatic isocyanides in an environmental friendly manner. The replacement of undesirable components such as phosphorous oxychloride, triethyl amine and dichloromethane (the classical combination used for the dehydration of N-formamides), by p-toluen sulphonyl chloride, sodium hydrogen carbonate and water makes this transformation really sustainable and safe.


 Please wait while we load your content...
Please wait while we load your content...