Stereoselective migratory heteroaryltrifluoromethylation of allylic amines via electrosynthesis†
Abstract
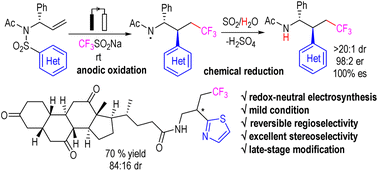
An electrochemically initiated heteroaryltrifluoromethylation of allylic amines was developed. The cascade reaction involved the anodic oxidation of Langlois reagent (CF3SO2Na) to generate CF3 radicals, followed by radical addition to the inactivated alkene, Smiles-type heteroaryl migration, and the reduction of nitrogen radicals. Unlike the conventional oxidative reaction mode, the SO2 molecules from the Langlois reagent and Smiles rearrangement probably served as chemical reductants in the reaction cascade, giving a redox-neutral electrosynthesis. The reaction exhibited excellent regioselectivity and stereoselectivity. It provides an efficient method for the synthesis of enantiomerically pure β-heteroaryl-γ-trifluoromethyl amines and the stereoselective late-stage modification of biologically active compounds.


 Please wait while we load your content...
Please wait while we load your content...