Electrochemical cascade synthesis of α-thio-substituted masked aldehydes†
Abstract
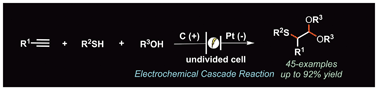
The recent upsurge in electrosynthesis has provided efficient and sustainable synthetic strategies for procuring valuable molecules and their precursors. In this context, we have achieved an efficient cascade synthesis of β,β-dialkoxy sulfides under ambient electrochemical conditions. The electrolysis of terminal aryl alkynes, aromatic and aliphatic thiols, and alcohols initiates a cascade sequence involving the thiol–yne reaction/1,2-thio migration via nucleophilic attack of alkoxides to provide α-thio-substituted masked aldehydes in good yields for a diverse range of substrates. The cascade sequence also works well when using acetic acid and acetic anhydride instead of alcohols.


 Please wait while we load your content...
Please wait while we load your content...