Electrooxidative tricyclic 6–7–6 fused-system domino assembly to allocolchicines by a removable radical strategy†
Abstract
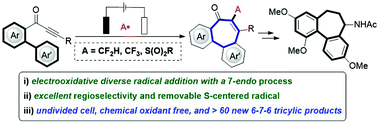
Natural allocolchicine and analogues derived thereof a tricyclic 6–7–6-system have been found as key scaffold of various biologically relevant molecules. However, the direct preparation of the allocolchicine motif remains difficult to date. Herein, we report on an electrooxidative radical cyclization of biarylynones with various carbon- and heteroatom-centered radical precursors via a sequential radical addition/7-endo-trig/radical cyclization domino reaction. This approach provides a step-economical and strategically novel disconnection for the facile assembly of a wide range of carbocyclic 6–7–6 fused ring systems. Remarkably, the sulfonyl group on the products could be easily removed by photocatalysis at room temperature with high yields.
- This article is part of the themed collection: 2022 Green Chemistry Hot Articles


 Please wait while we load your content...
Please wait while we load your content...