Electrochemical synthesis of α-amino amides via C(sp3)–H bond activation†
Abstract
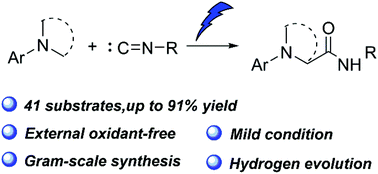
Multicomponent reactions provide a fast and efficient tool to construct various high-value molecules in the field of synthetic chemistry. Herein, we report a novel electrochemical oxidative four-component reaction for the synthesis of α-amino amides via C(sp3)–H bond activation. The active intermediate generated by the difunctionalization of isocyanide undergoes intermolecular rearrangement rather than the easier intramolecular rearrangement. This may be caused by a six-member ring transition state. In addition, under metal-free and external oxidant-free conditions, large-scale synthesis and good functional group tolerance indicated the potential applicability of this methodology.


 Please wait while we load your content...
Please wait while we load your content...