Reagent-free intramolecular hydrofunctionalization: a regioselective 6-endo-dig cyclization of o-alkynoylphenols†
Abstract
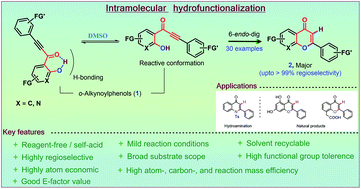
Solvent-directed intramolecular hydrofunctionalization of readily available o-alkynoylphenols 1 was successfully achieved under reagent-free conditions. The hydrofunctionalization of 1 occurred by nucleophilic attack on the phenolic oxygen followed by consecutive migration of the phenolic H atom to the alkyne center, eventually affording γ-benzopyranones 2. The phenol O–H group forms intramolecular H-bonds with the carbonyl group, and we predict that these H-bonds can be distorted into their most preferred conformation in the presence of polar solvents. A regioselective 6-endo-dig cyclization seems to be thermodynamically favoured over 5-exo-dig cyclization, as supported by DFT calculations. This strategy is remarkable because it is reagent-free, regioselective, highly atom economical, and highly atom, carbon and reaction mass efficient.


 Please wait while we load your content...
Please wait while we load your content...