Scalable selective electrochemical oxidation of sulfides to sulfoxides†
Abstract
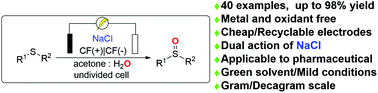
An electrochemical protocol for the selective oxidation of sulfides to sulfoxides has been developed in which NaCl plays a dual role: (1) as an electrolyte for the electrochemical transformations and (2) as a redox mediator to avoid oxidation of sensitive functional groups. Instead of a traditional oxidant, this methodology utilised traceless electrons as an ideal oxidant using anodic oxidation to access sulfoxides in good to excellent yields at ambient temperature. This metal-free electrochemical protocol is simple, environmentally friendly, and compatible with various sensitive functional groups using mixed acetone/water as the green solvent. Moreover, the cost-effective graphite felt electrodes can be reused up to ten times without loss of electrochemical activity. The methodology could be easily conducted on a gram or even a decagram scale.


 Please wait while we load your content...
Please wait while we load your content...