Divergent C(sp2)–H arylation of heterocycles via organic photoredox catalysis†
Abstract
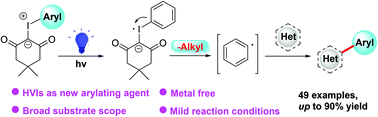
Introducing aryl moieties into heterocyclic scaffolds is a key step in the syntheses of natural products, drugs, and functional materials. Herein, we describe an unprecedented photocatalytic direct C(sp2)–H arylation of heterocycles. Notably, hypervalent iodine(III) ylides (HVIs) were firstly employed as a general arylating reagent under mild and easily handled reaction conditions, in which aryl radicals were afforded from the HVIs through single electron transfer (SET) processes in the presence of an organic dye as the photocatalyst. A wide scope of the substrates and an excellent tolerance of functional groups were attained in this transformation. Meanwhile, the efficient post modification of pharmaceutical molecules demonstrated its practicability.


 Please wait while we load your content...
Please wait while we load your content...