Multi-enzymatic cascade reactions with Escherichia coli-based modules for synthesizing various bioplastic monomers from fatty acid methyl esters†
Abstract
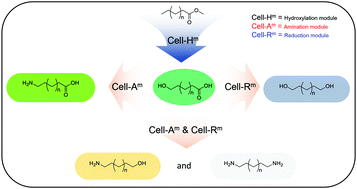
Multi-enzymatic cascade reaction systems were designed to generate biopolymer monomers using Escherichia coli-based cell modules, capable of carrying out one-pot reactions. Three cell-based modules, including a ω-hydroxylation module (Cell-Hm) to convert fatty acid methyl esters (FAMEs) to ω-hydroxy fatty acids (ω-HFAs), an amination module (Cell-Am) to convert terminal alcohol groups of the substrate to amine groups, and a reduction module (Cell-Rm) to convert the carboxyl groups of fatty acids to alcohol groups, were constructed. The product-oriented assembly of these cell modules involving multi-enzymatic cascade reactions generated ω-ADAs (up to 46 mM), α,ω-diols (up to 29 mM), ω-amino alcohols (up to 29 mM) and α,ω-diamines (up to 21 mM) from 100 mM corresponding FAME substrates with varying carbon chain length (C8, C10, and C12). Finally 12-ADA and 1,12-diol were purified with isolated yields of 66.5% and 52.5%, respectively. The multi-enzymatic cascade reactions reported herein present an elegant ‘greener’ alternative for the biosynthesis of various biopolymer monomers from renewable saturated fatty acids.


 Please wait while we load your content...
Please wait while we load your content...