One-step solvent-free aerobic oxidation of aliphatic alcohols to esters using a tandem Sc–Ru⊂MOF catalyst†
Abstract
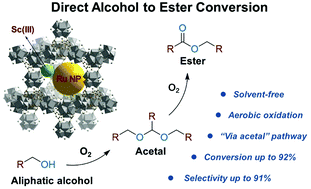
Esters are an important class of chemicals in industry. Traditionally, ester production is a multi-step process involving the use of corrosive acids or acid derivatives (e.g. acid chloride, anhydride, etc.). Therefore, the development of a green synthetic protocol is highly desirable. This work reports the development of a metal–organic framework (MOF) supported tandem catalyst that can achieve direct alcohol to ester conversion (DAEC) using oxygen as the sole oxidizing agent under strictly solvent-free conditions. By incorporating Ru nanoparticles (NPs) along with a homogeneous Lewis acid catalyst, scandium triflate, into the nanocavities of a Zr MOF, MOF-808, the compound catalyst, Sc–Ru⊂MOF-808, can achieve aliphatic alcohol conversion up to 92% with ester selectivity up to 91%. A mechanistic study reveals a unique “via acetal” pathway in which the alcohol is first oxidized on Ru NPs and rapidly converted to an acetal on Sc(III) sites. Then, the acetal slowly decomposes to release an aldehyde in a controlled manner for subsequent oxidation and esterification to the ester product. To the best of our knowledge, this is the first example of DAEC of aliphatic alcohols under solvent-free conditions with high conversion and ester selectivity.


 Please wait while we load your content...
Please wait while we load your content...