Selective hydrodeoxygenation of acetophenone derivatives using a Fe25Ru75@SILP catalyst: a practical approach to the synthesis of alkyl phenols and anilines†
Abstract
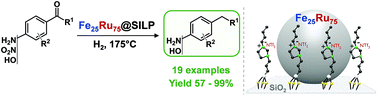
A versatile synthetic pathway for the production of valuable alkyl phenols and anilines has been developed based on the selective hydrodeoxygenation of a wide range of hydroxy-, amino-, and nitro-acetophenone derivatives as readily available substrates. Bimetallic iron ruthenium nanoparticles immobilized on an imidazolium-based supported ionic liquid phase (Fe25Ru75@SILP) act as highly active and selective catalysts for the deoxygenation of the side-chain without hydrogenation of the aromatic ring. The catalytic system allows operation under continuous flow conditions with high robustness and flexibility as demonstrated for the alternating conversion of 3′,5′-dimethoxy-4′-hydroxyacetophenone and 4′-hydroxynonanophenone as model substrates.


 Please wait while we load your content...
Please wait while we load your content...