One-pot production of phenazine from lignin-derived catechol†
Abstract
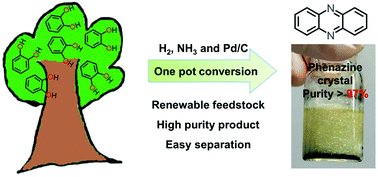
Upgrading lignin-derived monomeric products is crucial in bio-refineries to effectively utilize lignin. Herein, we report a simple strategy to convert catechol to phenazine, a useful N-heterocycle three-aromatic-ring compound, whose current synthetic procedure is complex via a petroleum-derived feedstock. The reaction uses catechol as the sole carbon source and aqueous ammonia as reaction media and a nitrogen source. Without additional solvents, phenazine was obtained in 67% yield in the form of high purity crystals (>97%) over a Pd/C catalyst after a one-pot-two-stage reaction. When cyclohexane was used as a co-solvent in the first step, a higher yield (81%) and purity (>99%) were achieved. Mechanistic investigations involving control experiments and an isotope labeling study reveal that hydrogenation, amination, coupling and dehydrogenation reactions are the key steps leading to phenazine formation. The conversion of other lignin-derived catechols highlights that the protocol is extendable to produce substituted phenazines.


 Please wait while we load your content...
Please wait while we load your content...