Separation of bio-based glucaric acid via antisolvent crystallization and azeotropic drying†
Abstract
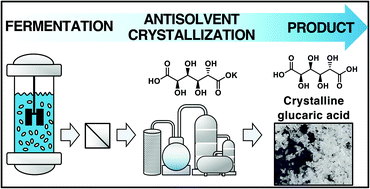
Glucaric acid is regarded as a top-value added compound from biomass, however, due to prevalent lactonization, the recovery of purified glucaric acid is challenging. Accordingly, an efficient method for glucaric acid separation, especially its diacid form, is necessary to facilitate its valorization. Here, we report a robust separation process that produces glucaric acid crystals from fermentation broth. This process first recovers purified monopotassium glucarate from broth and then recovers purified glucaric acid through acidification and antisolvent crystallization. Isopropanol was found to be an effective antisolvent reducing the solubility of glucaric acid while concomitantly forming an azeotrope with water. This allows solvent removal at low temperature through azeotropic drying, which avoids lactonization, and thus prevents impurities in the resulting crystals. Overall, this process was found to separate monopotassium glucarate and glucaric acid with a recovery yield of >99.9% and 71% at purities of ca. 95.6 and 98.3%, respectively. Process modeling demonstrates the ability to recycle the antisolvents IPA and acetone with >99% recovery and determined the energy input to be ∼20 MJ kg−1 for isolation of monopotassium glucarate and 714 MJ kg−1 for glucaric acid (0.06 M). The approach detailed in this work is likely applicable to the separation of other highly oxygenated bio-carboxylic acids (e.g., mevalonic acid) from fermentation broths, as well as to their recovery from abiotic reaction solutions.


 Please wait while we load your content...
Please wait while we load your content...