The need to change the approach to the safe use of herbicides by developing chiral and environmentally friendly formulations: a series of enantioselective (R)- and (S)-phenylethylammonium chloroacetates†
Abstract
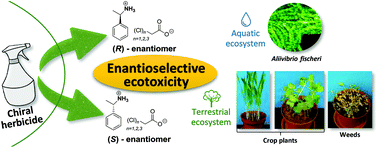
Most of the commercial chiral herbicides are used as racemic mixtures, whereas the use of their optically active forms may be more effective and environmentally safer. For this reason, it is important to evaluate the ecotoxicity of each enantiomer separately because a seemingly inactive enantiomer may unintentionally be toxic to non-target organisms. This research is focused on a series of chiral quaternary ammonium salts (QASs) consisting of (R)- or (S)-1-phenylethylammonium cations and chloroacetate anions, which were synthesized by a solvent-free green protocol. The enantioselective ecotoxicity towards non-target organisms, for the first time explored against aquatic bacteria Aliivibrio fischeri and terrestrial plants, was investigated for both (S)- and (R)-enantiomers. It has been shown that the obtained QASs exhibit enantioselective toxicity towards all tested organisms – up to a 9-fold difference in the R/S toxicity towards A. fischeri was observed. The correlation of toxicity with the amount of chlorine atoms in chloroacetate anions has been observed for the series of (R)-enantiomers (spring barley) and (S)-enantiomers (A. fischeri). Investigation of the ecotoxicity of racemic mixtures of the tested compounds, which was predicted using the CA model, showed that the use of racemic mixtures can have a much more negative impact on the environment than the use of enantiomerically pure formulations. Furthermore, by predicting the bioaccumulation risk (log Kow, bioconcentration factor) for the synthesized QASs, it has been demonstrated that commercial herbicides pose a greater tendency to be persistent in the environment than synthesized QASs.


 Please wait while we load your content...
Please wait while we load your content...