Cu@CuCl-visible light co-catalysed chlorination of C(sp3)–H bonds with MCln solution and photocatalytic serial reactor-based synthesis of benzyl chloride†
Abstract
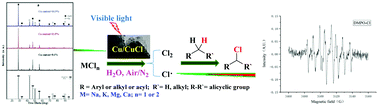
A highly selective, green and sustainable chlorination of aliphatic C–H bonds is reported using MCln under the co-catalysis of visible light and nano-Cu@CuCl, which was synthesised via a one-step, hydrothermal strategy using copper(II) sulfate and D-glucose as the reducing agent in a KCl solution. A novel cascade method of producing Cl2 occurs in situ chlorinating C(sp3)–Hs, achieved through nano-Cu@CuCl catalysis using inorganic chloride MCln as a chlorine source under visible light and without the need for a strong oxidant. The reaction was applied to the chlorination of different alkylarene α-Hs and cyclic hydrocarbons C–HS and was developed for use in a photocatalytic serial reactor system which accomplished the chlorination of toluene α-Hs to give 92% conversion, 90% yield and 98% selectivity. Electron spin resonance (ESR) studies of DMPO-Cl. revealed that the reaction mechanism belonged to a free radical process.


 Please wait while we load your content...
Please wait while we load your content...