Theaflavin-3,3′-di-gallate represses prostate cancer by activating the PKCδ/aSMase signaling pathway through a 67 kDa laminin receptor†
Abstract
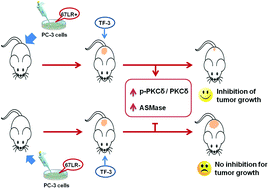
Prostate cancer is a major cause of morbidity and mortality in men. Theaflavin-3,3′-digallate (TF-3) is an important functional ingredient of black tea. We aimed to evaluate the cytotoxic effects of TF-3 on prostate cancer and to identify the underlying molecular mechanism. In this study, we explored the effects of TF-3 on prostate cancer in PC-3 cells and in NOD/SCID mice with prostate cancer. The results demonstrated that TF-3 inhibited prostate cancer cell proliferation by regulating the PKCδ/aSMase signaling pathway. The anti-prostate cancer effect of TF-3 was attributed to the expression of the 67 kDa laminin receptor (67LR), which is overexpressed in various cancers, playing a vital role in the growth and metastasis of tumor cells. Stable knockdown of 67LR could efficiently inhibit TF-3 induced apoptosis and cell cycle arrest in PC-3 cells, through interacting with the PKCδ/aSMase signaling pathway. In vivo studies also confirmed the above findings that TF-3 effectively inhibited tumor growth in terms of tumor volume. TF-3 treatment can significantly inhibit tumor growth and up-regulate the phosphorylation of PKCδ and the expression of aSMase in tumor xenografts developed by subcutaneously implanting PC-3 cells and 67LR-overexpressing PC-3 cells in mice. However, in tumor xenografts formed by subcutaneously implanting 67LR-knockdown PC-3 cells, TF-3 has no significant effect on PKCδ/aSMase pathway regulation and tumor growth inhibition.


 Please wait while we load your content...
Please wait while we load your content...