Rutin-loaded polymeric nanorods alleviate nephrolithiasis by inhibiting inflammation and oxidative stress in vivo and in vitro†
Abstract
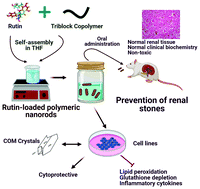
Polycrystalline aggregates formed in the glomerulus or other components of the urinary system represent the most critical step in kidney stone formation. The most common form of these crystals is calcium oxalate monohydrate (CaC2O4·H2O). Rutin is a potent antioxidant phytochemical, however, hydrophobicity and limited bioavailability restrain it from clinical applications. We developed a biocompatible amphiphilic triblock copolymer, PLGA–PEG–PLGA-loaded rutin nanorods, by simple and efficient self-assembly. Incorporation of polymer changed the topology of crystalline rutin into nanorods with non-Fickian sustained drug release kinetics by the Korsmeyer–Peppas model and thermodynamically non-spontaneous release of rutin. Rutin nanorods changed the growth and morphology of CaC2O4 crystals from the monohydrate to dihydrate form by increased adsorption and specific surface area from 0.8027 to 5.4233 m2 g−1, respectively. Rutin nanorods restored cell viability and oxidative stress in MDCK cells by modulating OPN expression and counteracts the proinflammatory signaling in THP-1 macrophages triggered by CaC2O4 crystals (80 μg cm−2). Rutin nanorods resulted in significant protection in serum and urinary biochemistry with reduced calcifications and increased tissue viability of kidneys without any toxicity and achieved high bioavailability. Our data provide a facile strategy for the use of rutin nanorods as a targeted drug system to treat and prevent renal stone formations.


 Please wait while we load your content...
Please wait while we load your content...